[Hui Cong Pharmaceutical Industry Network] A series of adjustments since the beginning of 2015 in the field of drug research and development. In 2016, the initial results have been seen: the number of approvals for drug declarations has decreased significantly, and the backlog of applications has gradually eased. At the same time, despite the increase in the speed of product approval, the number of newly approved drugs has also decreased significantly due to the large number of withdrawals.
According to the State Food and Drug Administration (CFDA) monthly approval of the listed drug announcement from January to November 2016, as of November 2016, there were only 192 drugs approved by the CFDA (according to the number of approvals, the same below), and 2014 More than 300 newly approved drugs were approved throughout the year and throughout 2015. According to the current approval process, the number of newly approved drugs in 2016 will be only about 2/3 of the previous two years.
Chemical drugs are still the most important category of drug research and development. Of the 192 varieties approved from January to November 2016, 175 are chemical drug varieties. The number of biological drugs remained basically stable, with a total of 12 approved varieties, but the number of approved Chinese medicines declined sharply, and only 5 varieties were approved.
Under the background of the state's efforts to strengthen Chinese medicine, the backwardness of the research and development of Chinese medicine is regrettable. Is it the problem of the enterprise's attention, the executable problem of the policy, or the problem of the product itself? These need Chinese medicine people to ponder.
Compared with previous years, the situation of low-level repeated declarations has been significantly alleviated, and more valuable and distinctive varieties have been approved, which will bring more benefits to the clinic.
The future value of the 2016 newly approved variety
For the 192 varieties approved in 2016, the author has taken the most sought after market value, the most anticipated by the patients, the most innovative value, the most expected by the parents, the most enjoyable two-child policy dividend, the most fun, and the most å°´å°¬7 The category "most" varieties.
1, the most sought after by the market
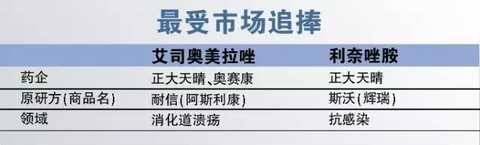
Esomeprazole (ie esomeprazole) is the best-selling drug in the field of digestion, as a proton pump inhibitor (PPI), which is widely used in a variety of digestive ulcer and hemorrhagic diseases. AstraZeneca's original research drug Nike's 2015 sample hospital sales reached 800 million yuan. Before 2016, in addition to the original research drug, only Laimei's oral generic drug Laimei Shu. In 2016, Zhengda Tianqing and Osei Kang's injection of Esomeprazole were approved at the same time. Considering that the PPI market for injection is larger, the two varieties are undoubtedly highly sought after by the market. In particular, Osei Kang, as a leading company in domestic PPI injections, will receive a huge sales increase for the approval of Esomeprazole Injection.
Linezolid is a synthetic oxazolidinone antibiotic. Despite being an antibiotic, linezolid, which is resistant to severely resistant infections, is highly promising even in the context of limited resistance. In 2015, the original research drug Pfizer's "Swar" sample hospital sales of nearly 70 million yuan, the first imitation drug Hausen's linezolid was only listed at the end of 2015. Considering the large number of patients with severe drug-resistant infections, linezolid has a huge market space, and the second generic drug approved in 2016 is also worth looking forward to.
2, the most expected by patients
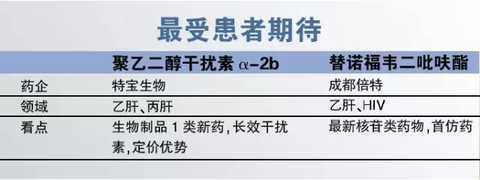

Although domestic treatment guidelines for hepatitis B and hepatitis C have clearly listed peginterferon alpha as a first-line drug, long-term interferon in China has long been monopolized by the original research drug, Roche's PEGASYS and Merck's PegIncano Price High, some patients can only choose long-term injection of short-acting interferon. Fortunately, in 2016, the domestic long-acting interferon alpha was finally approved for marketing. Peggy (polyethylene glycol interferon alpha-2b) is a national biopharmaceutical class 1 drug developed by Tebao Biotechnology and has completely independent intellectual property rights. In terms of pricing, Peibinbin is 20% to 30% lower than the long-acting α-2b, which can reduce the burden on patients to a certain extent.
Tenofovir, which is also used as a star of liver disease, is also a product of concern for patients with liver diseases. Tenofovir is currently the most excellent nucleoside drug in terms of drug resistance and negative conversion. Although tenofovir has entered China for many years, on the one hand, there are only HIV indications in the early stage, and on the other hand, the excessive price (the daily treatment cost exceeds 30 yuan) makes the market unable to expand for a long time. In view of this, patients expect that domestic tenofovir can be listed as soon as possible. In this context, Chengdu Bite successfully grabbed the first generic drug, and its price is expected to drop significantly compared to the original drug. At the same time, the original research drug “Weiride†also cut the price by more than 67% in the national drug negotiations. Some provinces and cities have begun to implement new prices. Two favorable factors will undoubtedly make more patients with hepatitis B choose tenofovir.
3, the most innovative value of domestic new drugs

The research and development of new molecules of chemical drugs is undoubtedly very difficult, but in 2016, a new class of new drug developed in China, nenofloxacin, was successfully approved. The Sinorafloxacin capsule (Taijiexin) jointly developed by Zhejiang Medicine and Taiwan Taijing Bio is a new type of quinolone drug. The antibacterial spectrum of this drug is broad and effective against drug-resistant bacteria, and it is safer without fluorine. Considering that the market promoter Zhejiang Pharmaceutical and Yuanfangfang Taijing Bio are all Chinese companies, this is also a model for the successful listing of domestic new drugs. But for the future to sell well, naloxacin needs more evidence-based research to support it.
GLP-1 drugs are the hotspot of global diabetes drug research in recent years. Six products have been successfully approved, and the best-selling liraglutide has annual sales of nearly 3 billion US dollars. At the end of 2016, Renai Bio-independent research and development of benaglutide was approved by CFDA and became the first domestically developed GLP-1 drug. Compared with traditional oral medication, GLP-1 drugs have strong hypoglycemic effect, low risk of hypoglycemia, and can reduce body weight. However, excessive treatment costs hinder their use in China. We expect benaglutide to significantly reduce GLP. The cost of treatment for -1 drugs will benefit more patients.
4, the most expected by parents

Vaccine is an important method to prevent pneumonia in children, and the best-selling vaccine in the world is Pei (Pneumococcal polysaccharide conjugate vaccine) for children with pneumonia. The 7-valent pneumococcal vaccine (Peer 7) has been on the market for many years, but the 7-valent vaccine has a slight deficiency in pneumococcal resistance with more than 90 serotypes. Therefore, the original research institute Pfizer developed an upgraded version of the 13-valent vaccine Pea 13 . However, while the new Pei 13 was declared, Pei 7 gradually stopped supplying, which made it possible for parents to go online and ask for help. After years of approval, Pei Er 13 has finally obtained Chinese approval, so the pneumonia vaccination problem that has plagued a large number of parents will be solved.
5, the most enjoy the two children policy dividend

In the context of a full liberalization of the two-children, the need to increase pregnancy opportunities through drug intervention has increased dramatically. Market opportunities are further expanded in response to infertility follicle stimulating hormone as a commonly used drug in this field. Follicle stimulating hormone is divided into urinary follicle stimulating hormone and recombinant follicle stimulating hormone. At present, the sales of follicle stimulating hormone in domestic sample hospitals exceeds 500 million yuan, but only 5 approved varieties. In the context of fully releasing the two children, the newly approved follicle stimulating hormone There will be greater market opportunities.
In 2016, Shanghai Tianwei Biological's urinary follicle stimulating hormone was approved. This drug is the third approved urinary follicle stimulating hormone in China and is expected to share the market dividend brought by the two-child policy.
6, the most fun
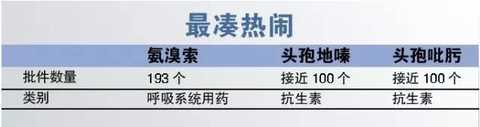
The phenomenon of getting together and reporting has been significantly alleviated in 2016. For many varieties that have been approved, new declarations have become rare. However, several new applications for ambroxol, cefodizime and cefepime have been approved.
Cefodizime for injection and cefepime for injection are currently close to 100, and the two newly approved varieties of Harbin Pharmaceutical and Suzhou Erye Pharmaceutical need to think more about how to get the opportunity from so many first entrants.
Ambroxol is more serious in repeated declarations. CFDA data shows that the approved batch of ambroxol has reached 193, of which 5 were contributed in 2016. The newly approved manufacturers can only show their brilliance from the already severely saturated market. Looking for opportunities.
7, the most embarrassing

The human papilloma vaccine, also known as the HPV vaccine, is currently the world's best-selling preventive vaccine for adults. It is widely believed that the vaccination of this vaccine will significantly reduce the incidence of HPV-related malignancies such as cervical cancer. However, the long-term HPV vaccine in the Mainland could not be approved, which also led some people to choose to vaccinate in Hong Kong or Japan and South Korea.
In 2016, GSK's bivalent human papillomavirus vaccination vaccine was finally approved in China. However, it is quite embarrassing that, just in the same period, the two-price HPV announced the termination of its sales in the United States. Similar to the pneumonia vaccine, HPV also includes a variety of subtypes. The marketed 2-valent vaccine is only effective for HPV types 16 and 18, which results in incomplete immunization. The fourth- and nine-valent vaccines with a larger immunization range are listed later. The scope of immunization is more reliable than the two-valent vaccine. Although the two-valent vaccine can still greatly reduce the risk of infection, the delisting of the United States undoubtedly makes many people who are ready to inject a doubt about its efficacy and safety.
Expected varieties that 2017 is expected to be listed
With the implementation of the priority review system, the differentiation of drug approval speeds is becoming more and more obvious. The state encourages the development of new drugs with higher levels of innovation and clinical value, especially for diseases of specific populations such as difficult diseases, rare diseases and elderly children. Special medicine. In addition, for the first generic drug, the review progress will also speed up, which indicates that more varieties worthy of expectation will be approved in 2017.
For the varieties that are worth looking forward to in 2017, we divide them into three categories: foreign innovative drugs, independent innovative drugs and high-level generic drugs.
1. Foreign innovative drugs
In 2017, it is estimated that there are not many foreign innovative drugs approved in China, among which the following four varieties are worthy of attention.
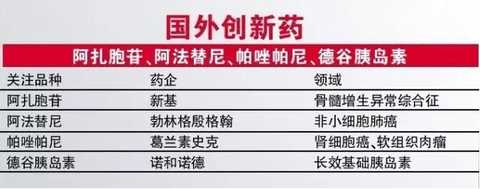
The new base of azacitidine is the world's first approved drug for myelodysplastic syndrome (MDS). The launch of this variety in China will undoubtedly further enhance the product advantages of the new base in the blood field. A study also demonstrated the existence of azacitidine in combination with lenalidomide.
Boehringer Ingelheim's TKI drug, afatinib, is a multi-targeted kinase inhibitor that acts on HER2 and irreversibly inhibits EGFR. It has been found that this drug can significantly improve non-existence compared with Iressa. The therapeutic effect of small cell lung cancer, so the drug obtained the FDA breakthrough identification. Considering the large number of lung cancer populations in China, afatinib has huge market opportunities.
Pazopanib (Pizozampir) is a TKI drug developed by GSK for renal cell carcinoma and soft tissue sarcoma. A study in the New England Journal suggests that pazopanib is similar to sunitinib in the treatment of renal cell carcinoma. But the safety is better, and pazopanib is also considered to be a better treatment for soft tissue sarcoma.
Compared with the first three oncology drugs, the author is more concerned about the development of insulin in Degu. Degutin is another long-acting basal insulin after insulin glargine and insulin detemir. Compared with insulin glargine, insulin glutathione has a lower risk of hypoglycemia and is convenient for clinical use (the insulin dose is 8 to 40 hours apart without affecting its effectiveness and safety). The original researcher hopes to use its challenge to obtain The dominance of the time.
2. Domestic innovative drugs
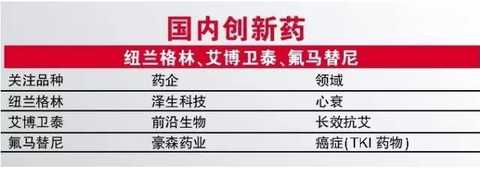
For Newland Green and Aibo Weitai, it seems that they are only a short walk from the market, but it seems to be far behind. Both first-in-class varieties have already completed clinical research and declared production. Newland Green is a drug for heart failure, and its mechanism is different from existing drugs. Aiboweitai is considered to be the first long-acting anti-AIDS drug. From the point of view of innovation and clinical value, both drugs are extremely valuable. Once approved, they will undoubtedly become the representative of new drug innovation in China, but precisely because both varieties belong to first-in-class, it can If we are approved, we can only show cautious optimism.
TKI is a hot spot for innovative drugs in China, and the listing of ectinib and apatinib has also proved that TKI drugs can be successfully developed in China. Hausen's flumatinib is currently in the production stage, the target of which is Bcr-Abl, which can be used in patients with Gleevec resistance, a treatment with slow-acting flumatinib and imatinib A randomized controlled trial showed that the effect of flumatinib was better.
3, high level generics
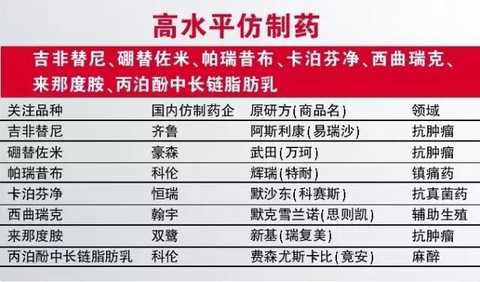

In addition to innovative drugs, we are equally concerned about some generic drugs that are expected to be approved in 2017. According to the list of priority reviews, we are optimistic about some of the best-selling drugs in the catalogue, such as gefitinib in Qilu, bortezomib inhausen, parecoxib in Cologne and caspofene in Hengrui . Other varieties worthy of expectation include Shenzhen Hanyu's first imitation drug, Citrix, the first imitation of lenalidomide and the new anesthetic propofol in medium-long-chain fat emulsion. .
Editor in charge: Yan Wenqian
Others Underwear,Lace Underwear Set,Red Underwear Set,Cute Underwear Sets
HANGZHOU TOSHENG TRADING CO.,LTD , https://www.tongshengtrading.com
